ascl3 electron geometry|AsCl3 Molecular Geometry : Baguio Arsenic trichloride is an inorganic compound with the formula AsCl3, also known as arsenous chloride or butter of arsenic. This poisonous oil is colourless, although impure samples may appear yellow. It is an intermediate in the manufacture of organoarsenic compounds. 1 PHP to USD stats. The performance of PHP to USD in the last 30 days saw a 30 day high of 0.0178 and a 30 day low of 0.0173. This means the 30 day average was 0.0176. The change for PHP to USD was 2.42. The performance of PHP to USD in the last 90 days saw a 90 day high of 0.0178 and a 90 day low of 0.0170. This means the 90 day average was .
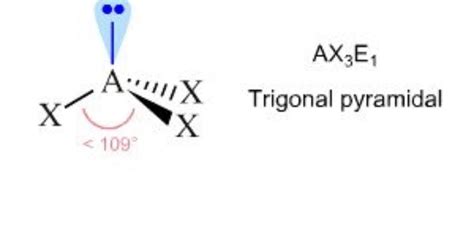
ascl3 electron geometry,The answer is B) trigonal pyramidal. To determine the molecular geometry of arsenic trichloride, AsCl_3, you must take a look at its Lewis structure. One arsenic trichloride molecule will have a total of .Arsenic trichloride is an inorganic compound with the formula AsCl3, also known as arsenous chloride or butter of arsenic. This poisonous oil is colourless, although impure samples may appear yellow. It is an intermediate in the manufacture of organoarsenic compounds.

Determine the Electron geometry from the Lewis dot structure. Determine the molecular geometry. It is very important from the onset that students understand the difference .
A step-by-step explanation of how to draw the AsCl3 Lewis Dot Structure ( Arsenic trichloride).For the AsCl3 structure use the periodic table to find the tot.
Solution. Verified by Toppr. Correct option is B. trigonal pyramidal. The answer is B) trigonal pyramidal. To determine the molecular geometry of arsenic trichloride, A s C l 3, you .
Hence, the valence electron present in chlorine is 7 (see below image). Hence in a AsCl3 molecule, Valence electrons given by Arsenic (As) atom = 5. Valence .Learning Objectives. To use the VSEPR model to predict molecular geometries. To predict whether a molecule has a dipole moment. The Lewis electron-pair approach can be . AsCl3 Molecular Shape + Lewis Dot Structure - YouTube. chem101csub. 3.77K subscribers. 15. 7.5K views 10 years ago. Chemistry learning made easy. This . Periodic table. In the periodic table, arsenic lies in group 15, and chlorine lies in group 17. Hence, arsenic has five valence electrons and chlorine has seven valence . Step 3: Connect each atoms by putting an electron pair between them. Now in the AsCl3 molecule, you have to put the electron pairs between the arsenic atom (As) and chlorine atoms (Cl). This .
Step 1. Given:- AsCl3. As -electronic configuration= [Ar] 3d¹⁰ 4s² 4p³. View the full answer Answer. Unlock. Previous question Next question.AsCl3 Molecular Geometry Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms, having a chemical formula of PCl3. It is a volatile liquid that reacts with water and releases HCl gas. It is a toxic .ascl3 electron geometry AsCl3 Molecular Geometry Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms, having a chemical formula of PCl3. It is a volatile liquid that reacts with water and releases HCl gas. It is a toxic . Here, V (valence electron of central atom) = 5 M ( monovalent atom) = Cl = 3 As it is a neutral compound thus C and A will be 0. Thus , H= ½ [5+3] = ½ * 8 = 4 = Sp3. These two concepts clearly explain the Sp3 hybridization of PCl3. PCl3 Molecular Geometry. The molecular geometry of PCl3 is a trigonal pyramid.The Electron Pair Geometry of a molecule is determined by the total number of electron pairs around a central atom. Electron pairs are the bonded electrons, lone pairs and single unpaired electrons. Total number of electron pairs = ½ X [(number of electron pairs on central atom) + (number of monovalent atoms on the central atom) + (anionic .
A step-by-step explanation of how to draw the AsCl3 Lewis Dot Structure ( Arsenic trichloride).For the AsCl3 structure use the periodic table to find the tot.
Question: 10) Determine the electron geometry (eg) and molecular geometry (mg) of AsCl3. A) eg=tetrahedral, mg-tetrahedral B) eg-linear, mg-trigonal planar C) eg=trigonal planar, mg=bent D) eg=linear, mg=linear E) eg=tetrahedral, mg=trigonal pyramidal
Table 1.1 Basic VSEPR Shapes. Notes: . For VSEPR purpose, the terms “shape” and “geometry” are interchangeable; “electron pair” and “electron group” are also interchangeable. Multiple bonds (double or triple bond) are regarded as one electron group for VSEPR purpose.; For species that do not have any lone pair electrons (LP), the .The Lewis structure for AsCl 3 is similar to AsF 3 structure. Since they are in the same Group on the periodic table they each have the same number of electrons their structures are similar. The Arsenic atom goes in the center of the Lewis structure since it is the least electronegative atom. For the AsCl 3 Lewis structure there are a total of .ascl3 electron geometryFigure 10.2.2 ): (CC BY-NC-SA; anonymous) The two oxygens are double bonded to the sulfur. The oxygens have 2 lone pairs while sulfur had one lone pair. 3. There are two bonding pairs and one lone pair, so the structure is designated as AX 2 E. This designation has a total of three electron pairs, two X and one E.1. The central atom, beryllium, contributes two valence electrons, and each hydrogen atom contributes one. The Lewis electron structure is. 2. There are two electron groups around the central atom. We see from Figure .Predict the electron pair geometry (EPG) and molecular geometry (MG) of the following compounds based on the Lewis structure and VSEPR: A. H2O B. PF5 C. CO2 D. SO2 E. CHCl3; Use the VSEPR method to predict the geometry of the following ion and molecules: a. OF_2. b. SiF_4. Using VSEPR theory predict the most likely structure for ClO_2.
The Lewis structure for AsClz has a total of 10 lone pairs. The central atom (As) has 4 electron groups around it. The Lewis structure for AsCl3 has 3 bonding pairs. The electron geometry around the central atom, As is trigonal pyramidal. The central atom, As has one lone pair. ΟΟ none of the above. An explanation of the molecular geometry for the SCl2 (Sulfur dichloride) including a description of the SCl2 bond angles. The electron geometry for the Sulf. Electron domain is used in VSEPR theory to determine the molecular geometry of a molecule. The convention is to indicate the number of bonding electron pairs by the capital letter X, the number of lone electron pairs by the capital letter E, and the capital letter A for the central atom of the molecule (AX n E m).When predicting .Molecular geometry is a way of describing the shapes of molecules. It applies a theory called VESPR for short. VESPR stands for valence shell electron pair repulsion. This theory basically says that bonding and non-bonding electron pairs of the central atom in a molecule will repel (push away from) each other in three dimensional space and this .
The central atom in AsCl3 is arsenic (As). First, we need to determine the electron geometry, which is the arrangement of electron groups (bonding and non-bonding pairs) around the central atom. Arsenic has 5 valence electrons, and each chlorine atom contributes 7 valence electrons. So, the total number of valence electrons in AsCl3 .

Arsenic trichloride is a chloride of arsenic prepared by the treatment of arsenic (III) oxide with concentrated hydrochloric acid, followed by distillation. It is used in preparation of many chloroderivatives of arsenic that have pharmaceutical and insecticide applications. Arsenic is a chemical element that has the symbol As and atomic number .
ascl3 electron geometry|AsCl3 Molecular Geometry
PH0 · What is the molecular geometry of AsCl3 ? A) tetrahedral B
PH1 · What is the molecular geometry of AsCl3 ? A)
PH2 · What is the molecular geometry of AsCl
PH3 · Lewis Structure of AsCl3 (With 6 Simple Steps to Draw!)
PH4 · AsCl3 Molecular Shape + Lewis Dot Structure
PH5 · AsCl3 Molecular Geometry
PH6 · AsCl3 Lewis structure
PH7 · AsCl3 Lewis Structure: How to Draw the Lewis Dot Structure for
PH8 · Arsenic trichloride
PH9 · 8.6: Molecular Geometries
PH10 · 10.2: VSEPR Theory